Trusted by








































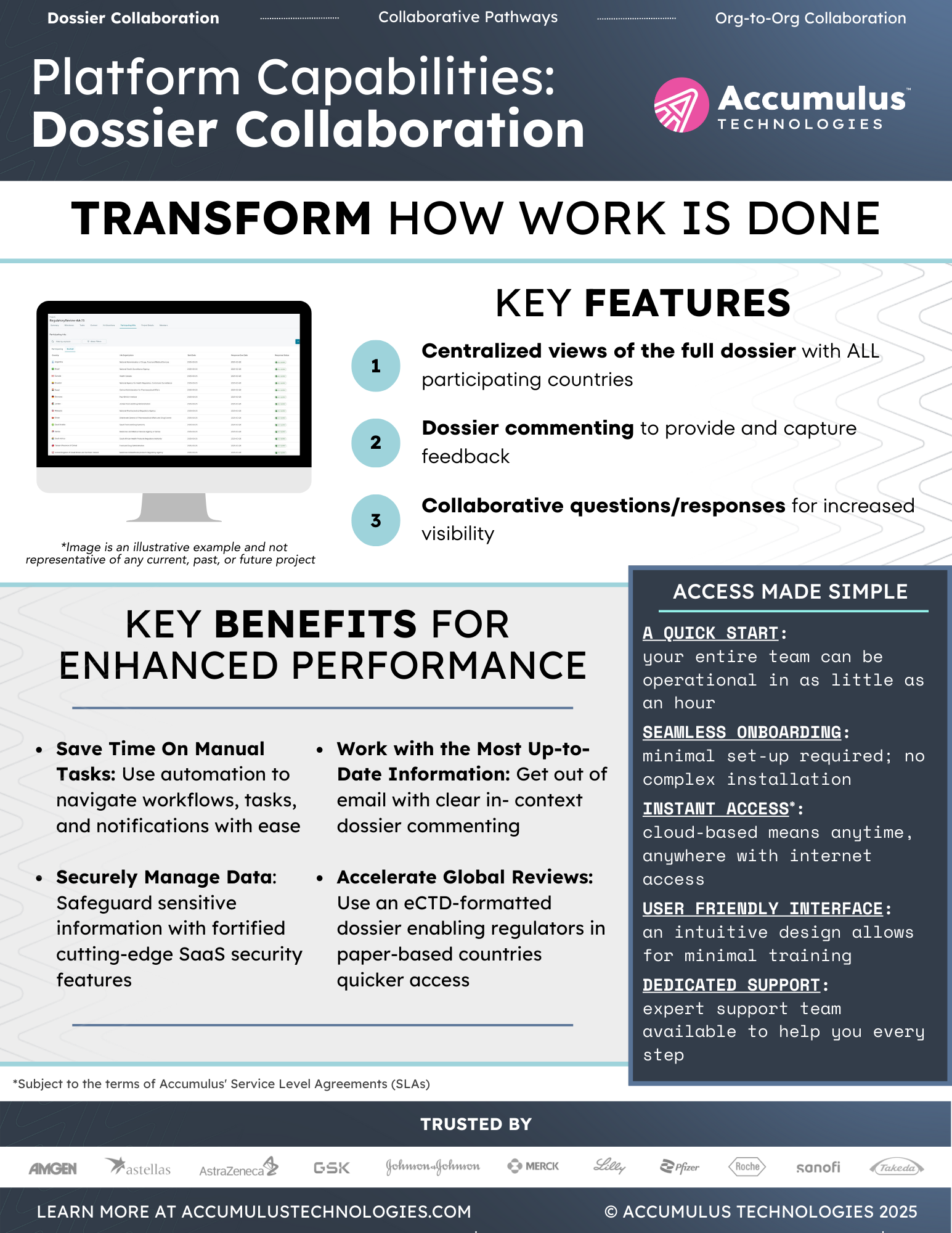
Healthcare Can’t Afford To Stay Disconnected
Drug development timelines continue to rise with information trapped in static documents and scattered across disconnected systems.
13 Years
Average time from initial discovery through regulatory approval for one new drug.
2 Million
Average number of pages of paper for one new drug application (the pile would be 200 meters tall).
$2 Billion
Average spend from initial discovery to regulatory approval to bring one new drug to market1.
1. Nurturing growth: measuring the return from pharmaceutical innovation. 2023. Deloitte. Available here.
"It’s no longer acceptable for information to remain siloed across the healthcare ecosystem. We’re living in an era of data abundance and AI-driven possibility — yet regulatory exchange remains slow, fragmented, and disconnected. Accumulus Technologies is leading an information revolution, creating the infrastructure to transport, visualize, and share critical information in real time. We're breaking down barriers between regulators, sponsors, and innovators — so that every contributor and every decision-maker is connected in service of one outcome: faster access to therapies for patients around the world."

FRANCISCO NOGUEIRA
Chief Executive Officer, Accumulus Technologies
A BETTER WAY
No Silos.
No Bottlenecks.
Just Connection.
Wherever you are in the drug development lifecycle, the Accumulus platform simplifies how you submit, review, or collaborate so that your regulatory information can flow securely and efficiently — from drug discovery to post approval.
We aren’t just moving information — we’re moving decisions, timelines, and outcomes.
WHY ACCUMULUS TECHNOLOGIES
Your needs, simplified
Speed
Faster decisions. Shorter timelines. Quicker access to life-changing therapies for patients around the world.
Savings
Unlock measurable cost benefit by streamlining submissions and reducing the cost of regulatory delays.

Scalability
Future-proof your digital strategy with a single platform that grows with you — across teams, markets, and product lifecycles.
Complex global submissions. Disconnected systems. Endless rework. We’ve simplified it all — so you can focus on advancing innovation.
Avoid typical frustrations and delays with traditional platforms. With the Accumulus platform you get:
A quick start: get your entire team up and running in as little as just one hour
Seamless onboarding: minimal set-up required- no complex installations or lengthy configuration
A user-friendly interface: intuitive design ensures that your team can start using the platform with minimal training
Instant access: cloud-based means accessible anytime, anywhere with internet connection
Dedicated support: expert support team available to help you every step of the way
Subject to the terms of Accumulus' Service Level Agreements (SLAs)